A federal judge in Texas on Friday ruled to suspend the abortion drug mifepristone, which was approved by regulators 23 years ago and has now become one of the most common methods of abortion in the country.
U.S. District Judge Matthew Kacsmaryk ruled to suspend the FDA's approval of mifepristone. The ruling is paused for seven days so the federal government may appeal.
Attorney General Merrick Garland said the Department of Justice strongly disagreed with the decision and would file an appeal.
"Today's decision overturns the FDA's expert judgment, rendered over two decades ago, that mifepristone is safe and effective. The Department will continue to defend the FDA's decision," Garland said in a statement.
Kacsmaryk, a Trump appointee, held a hearing on the issue on March 15 in Amarillo, Texas, where the conservative plaintiffs in the case argued the Food and Drug Administration was wrong to approve mifepristone.
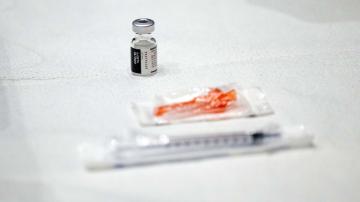
In this Jan. 13, 2023, file photo, a patient prepares to take mifepristone, the first pill given in a medical abortion, at a reproductive clinic in New Mexico.
Evelyn Hockstein/Reuters, FILE
Meanwhile, a Washington state judge issued a dueling injunction on Friday in a separate case regarding the status of the FDA's approval of mifepristone. In his ruling, Judge Thomas Rice of Washington's Eastern Federal District Court granted the preliminary injunction requested by the plaintiffs, made up of Democratic attorneys general, and prevents the FDA from "altering the status quo and rights as it relates to the availability of mifepristone."
GenBioPro, the manufacturer of generic mifepristone, responded to Kacsmaryk's ruling and said they were confident in the legality of the drug.
"Nothing in the court's order changes the decades of science and evidence regarding mifepristone's safety and efficacy. As we review the court's order, we will continue to make our product available," Evan Masingill, CEO of GenBioPro, said in a statement.
Masingill added that GenBioPro was reviewing Rice's order but was "pleased that he recognizes the importance of preserving the status quo as it relates to the availability of mifepristone."
About half of all abortions in the U.S. were medication abortions as of 2020, according to data collected by the Centers for Disease Control and Prevention. Such methods usually rely on mifepristone in a two-drug regimen along with misoprostol.
Erik Baptist, senior counsel with Alliance Defending Freedom, the group that filed the Texas lawsuit, said after the March hearing that the judge needed to provide a check on the FDA, which he said ignored safety concerns with mifepristone -- an allegation the government and most medical doctors refute.
In this Jan. 13, 2023, file photo, boxes of mifepristone, the first pill given in a medical abortion, are prepared for patients at Women's Reproductive Clinic of New Mexico in Santa Teresa, N.M.
Evelyn Hockstein/Reuters, FILE
"The FDA never had the authority to approve these drugs and remove important safeguards," Baptist said.
Government lawyers defending the FDA said at the hearing that the government has reviewed extensive data and found no such safety concerns.
"The public interest would be dramatically harmed" by siding with the plaintiffs, said Julie Straus Harris, an attorney for the Justice Department, while Baptist urged the judge: "Relief must be complete and nationwide."
Outside, pro-abortion access advocates were blunt. "There are a whole host of reasons why this court should just dismiss [the lawsuit] out of hand," said Carrie Flaxman, senior director for public policy litigation and law with Planned Parenthood Federation of America.
The FDA is appealing the ruling, standing behind its determination that the drug is safe and effective.
"Patients should have access to FDA-approved medications that FDA has determined to be safe and effective for their intended uses," the FDA said in a statement.
Danco Labs, which makes the brand name version of mifepristone and joined the FDA as a defendant in the lawsuit, called the ruling a "dark day in public health," in a statement. It said it's appealing the decision alongside the FDA.
If the conservative 5th Circuit Court of Appeals upholds the Texas ruling -- and the two federal orders remain in conflict -- the issue is likely to be expedited to go before the Supreme Court.
Alexis McGill Johnson, president and CEO of Planned Parenthood Federation of America, said in a statement to ABC News that the judge's decision in Texas "could threaten the FDA's role in this country's public health system, and -- if allowed to stand -- will have broad and unprecedented consequences that reach far beyond abortion."
The decision "is an outrage and exposes the weaponization of our judicial system to further restrict abortion nationwide. However, I want to be clear that access to mifepristone remains safe for now," the statement said. "But we should all be enraged that one judge can unilaterally reject medical evidence and overrule the FDA's approval of a medication that has been safely and effectively used for more than two decades."
Used boxes of Mifepristone pills, the first drug used in a medical abortion, fill a trash at Alamo Women's Clinic in Albuquerque, N.M., Jan. 11, 2023.
Evelyn Hockstein/Reuters, FILE
The American College of Obstetricians and Gynecologists condemned the Texas decision in a statement Friday and called it a "grievous legal overstep into America's well-established regulatory system."
"The decision itself betrays the bias and prejudice that informed its rhetoric, which deliberately ignores decades of evidence-based scientific data and eschews clinically appropriate language about mifepristone, a critical medication used for both abortion and miscarriage management," Dr. Iffath Abbasi Hoskins, the organization's president, and Dr. Maureen Phipps, its CEO, said in a joint statement.
Alliance Defending Freedom meanwhile said the decision Friday in its lawsuit is a "significant victory for the doctors and medical associations we represent and more importantly, the health and safety of women and girls."
Vice President Kamala Harris offered the first reaction from the Biden administration on the Texas ruling, telling reporters Friday night as she prepared to depart Nashville, Tennessee, that it sets a "dangerous precedent."
She and President Joe Biden will "stand with the women of America and do everything we can to ensure that women have the ability to make decisions about their health care, their reproductive health care," Harris said. "And they decide that, not their government."
ABC News' Justin Gomez and Meredith Deliso contributed to this report.